Of laser-active ions in a laser gain medium. You can look up that answer from the table.
Molarity Calculations Practice Khan Academy

Molarity Molar Concentrations Youtube
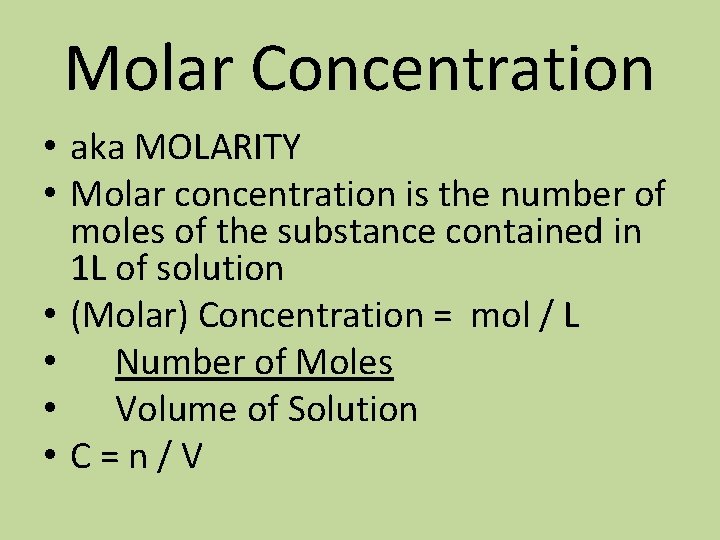
Molar Concentration Knowing The Concentration Of A Solution
Encyclopedia letter D doping concentration.

Molar concentration formula. Molarity is the concentration of x moles of solute in 1 L of solution. Divide the total moles of solute by the total volume of the solution in liters. The units of molar concentration are moles per cubic decimeterThey are noted as moldm³ as well as M pronounced molar.
It tells us how much a solute is present in a. Molecular weight is the mass of one molecule of a substance while the molar mass is the mass of one mole of a substance. In the case of glucose a 1 M solution would have 1 mole 180 g dissolved in 1 L.
This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole. You may be wondering why the molar mass of sodium isnt just twice its atomic number the sum of the protons and neutrons in the atom which would be 22. A 1 M solution is one in which exactly 1 mole of solute is dissolved in a total solution volume of exactly 1 L.
To convert molar to percentage concentration we. A concentration can be any kind of chemical mixture but most frequently solutes and solvents in solutions. The calculator will determine the amount of compound to add to a solution to achieve the desired molarity.
To do this calculate the empirical formula mass and then divide the compound molar mass by the empirical formula mass. Molar concentration also called molarity amount concentration or substance concentration is a measure of the concentration of a chemical species in particular of a solute in a solution in terms of amount of substance per unit volume of solution. The balanced chemical equation can be framed as HCl NaOH NaCl H 2 O.
Compound Name Formula Search Moles to Grams Calculator Common Compounds List Chemical Equation Balancer Complete List of Acids Complete List of Bases Molar to Mass Concentration Converter Molar Mass Calculator Cations Anions List Dilution Calculator Molarity Calculator Compound Prefixes Water Insoluble. You can use the empirical formula to find the molecular formula if you know the molar mass of the compound. Here is the simple online molar concentration calculator to calculate the molarity substance which is expressed as molL.
Calculate the concentration of ceF- in the solution and its absolute uncertainty. M 3 ppm. Enter the molecular weight desired molarity and desired final volume.
The standard equation for absorbance is A ɛ x l x c where A is the amount of light absorbed by the sample for a given wavelength ɛ is the molar absorptivity l is the distance that the light travels through the solution and c is the concentration of the absorbing species per unit volume. C is the molar concentration in molL Molar or M. Molar Concentration Formula Definition of Molar Concentration.
For an acid. This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solutionMolarity is defined as the number of moles of solute dissolved per liter of solution molL M. Its unfortunate they have been used for two quite different things.
Suggest additional literature. How to cite the article. This gives you the ratio between the molecular and empirical formulas.
NHCl 350 1000 dm 3 0250 mol dm-3. Multiply the relative atomic mass by the molar mass constant. µL 01 mL then this protein products mass concentration will be 20 µg 01 mL 200 µgmL.
In chemistry the most commonly used unit for molarity is the number of moles per liter having the unit symbol molL or moldm 3 in SI unit. C m C_m C m - molar concentration C p C_p C p - percentage concentration d d d - density of solution M M M - molar mass of substance. Optical materials laser devices and laser physics.
The concentration of some dopant eg. If the MW Molecular Weight of the protein is 40 KD then the molar concentration for this protein product is 200 µgmL 40 KD 5 µM. Mass concentrationlike molar concentration molality mass fraction is a quantity to measure the concentration of a solute in the solution.
Molarity calculation is used in teaching laboratory study and research. To find the molar concentration of a solution use the concentration formula. Analyze graph and present your scientific work easily with GraphPad Prism.
Formula of Molar Concentration. Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. The concentration of a solution of 1 mole dissolved in 1 L is 1 M one molar Dont confuse this symbol M for moll with M for molar mass.
Molarity is the number of moles of the solute dissolved per liter of the solution. Understand the Beer-Lambert law for absorbance A ɛ x l x c. Let me make it more clear with an example of sodium chloride.
From molar concentration to mass concentration. Though there are many methods by which to report the concentration molarity M is one of the most common and has units of moles per liter. Mass concentration molar concentration number concentration and volume concentration.
Using Empirical Formula to Find Molecular Formula. The molar mass links the mass of a substance to its moles. First of all uncertainty aside I got 00141 for the concentration of ceF-.
Solutions with varied molarities have different properties ie a low molarity acid and high molarity acid can. The molar mass of sodium metal is the mass of one mole of Na. It is the ratio of the mass of a solute present in the solution to the volume of the solution.
Molar concentration is the most convenient method of expressing the concentration of a solute in the given solution. Several types of mathematical description can be distinguished. It is defined as the number of moles of solute dissolved in a liter of solution and formula is defined as mv x 1MW.
In chemistry concentration is the abundance of a constituent divided by the total volume of a mixture. Thus M mol per L. The molar mass of.
The simple formula is. However this is not the correct answer according to Sapling Learning. The molar concentration of solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute eg the concentration of hydroxide anions can be written as OH.
The molar concentration formula is given by Solved Examples. The units for molecular mass are given in atomic mass units while the units for molar mass are give in grams per mol. This is defined as 0001 kilogram per mole or 1 gram per mole.
Determine the molar concentration of NaOH for the reaction between HCl and NaOH. This tool will calculate the molarity of a solution for a compound of known molecular weight.
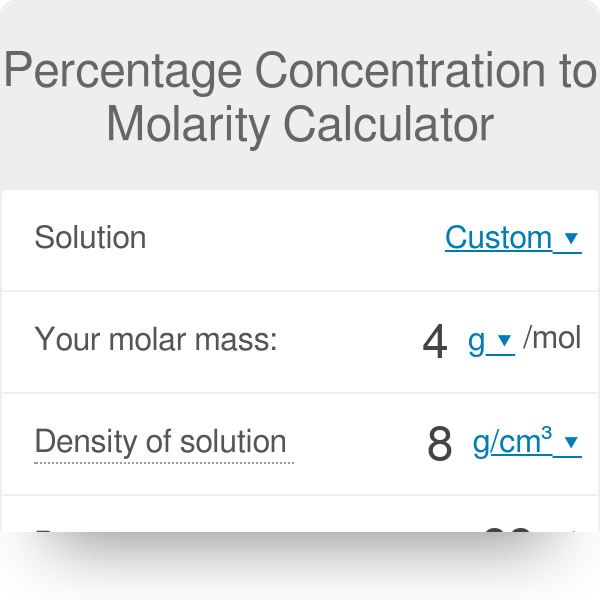
Percentage Concentration To Molarity Calculator
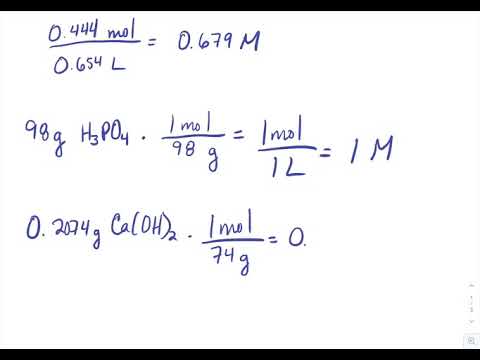
6 1 Calculating Molarity Problems Chemistry Libretexts

Molar Concentrations And Solutions

How To Calculate Molarity
Normality

5 Easy Ways To Calculate The Concentration Of A Solution
Tutortag Calculating Molar Concentration Using Formula Facebook

5 Easy Ways To Calculate The Concentration Of A Solution


